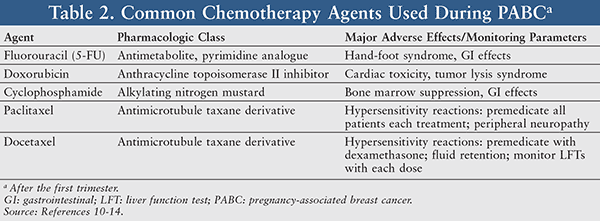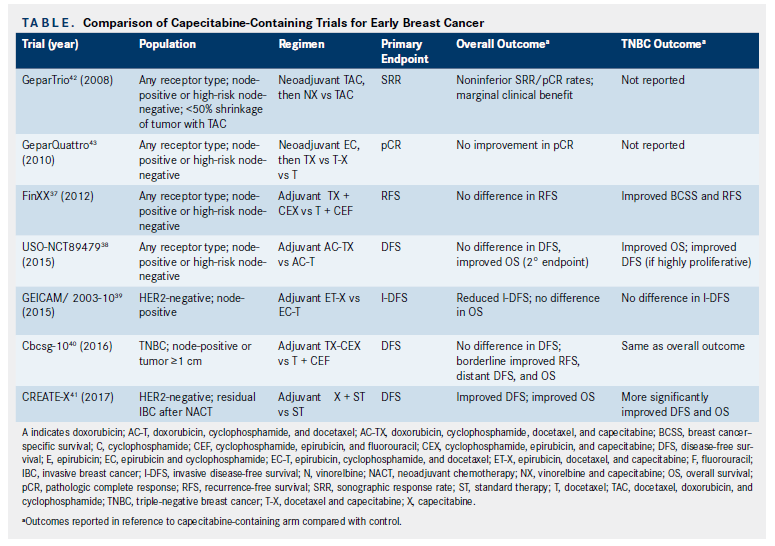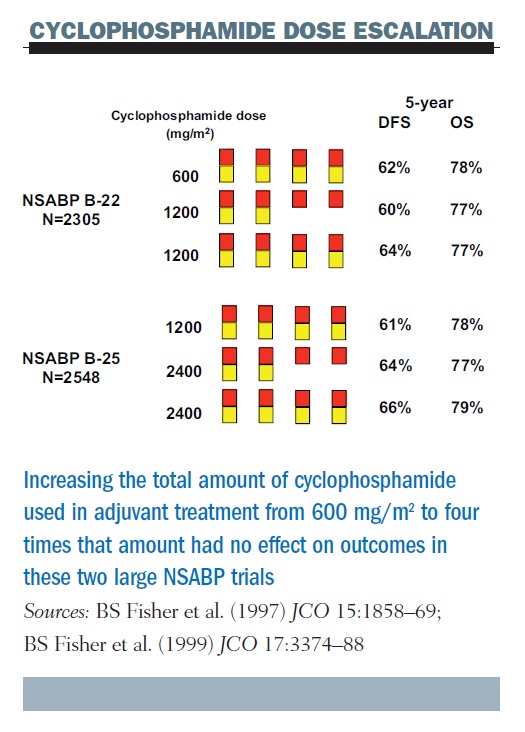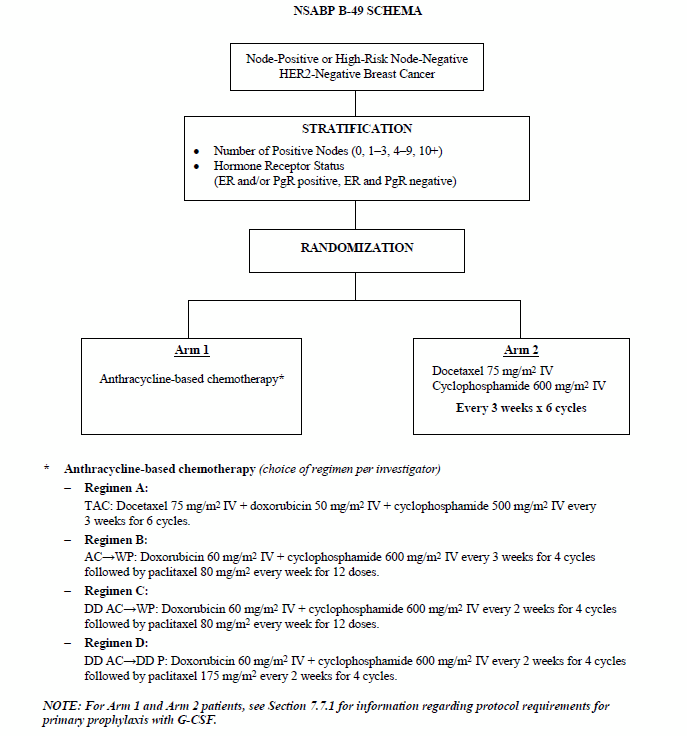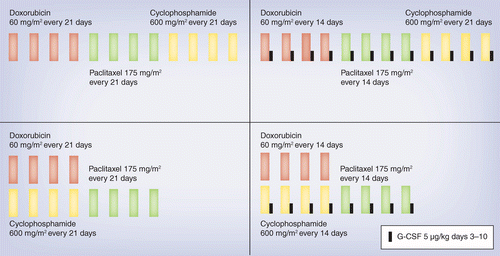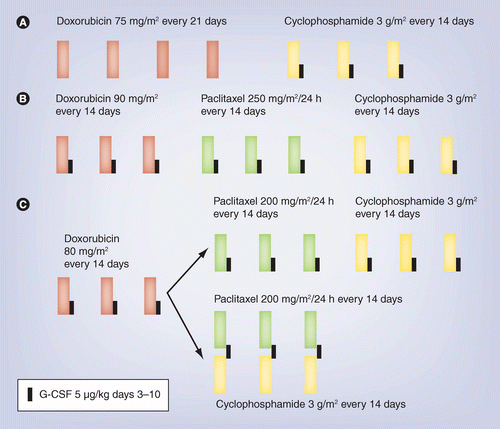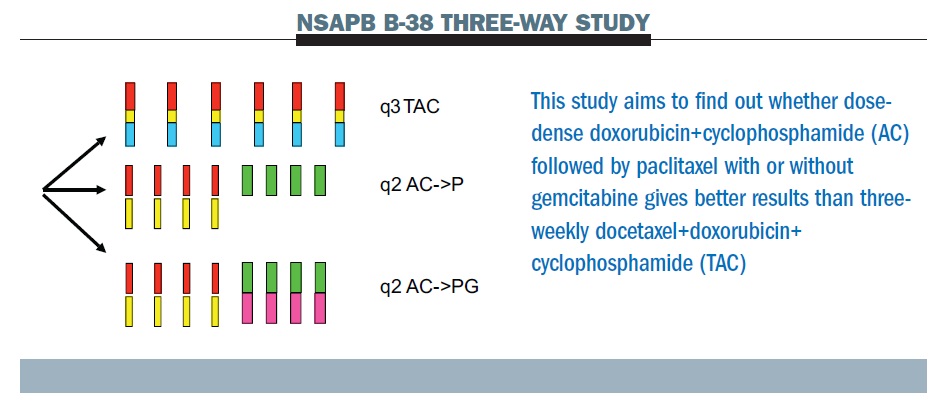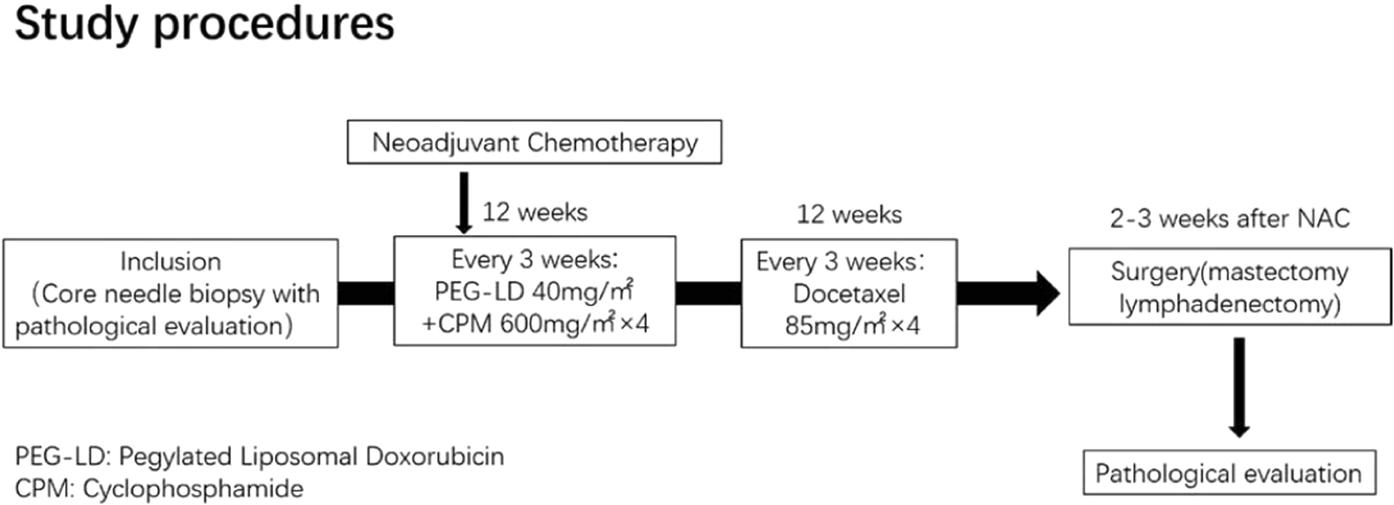
Pegylated liposomal doxorubicin plus cyclophosphamide followed by docetaxel as neoadjuvant chemotherapy in locally advanced breast cancer (registration number: ChiCTR1900023052) | Scientific Reports

PDF) Dose Dense Doxorubicin and Cyclophosphamide Followed by Paclitaxel (Dose Dense AC Followed by T) Regimen for Breast Cancer

Four cycles of docetaxel and cyclophosphamide as adjuvant chemotherapy in node negative breast cancer: A real-world study - ScienceDirect

Anthracycline-free or short-term regimen as adjuvant chemotherapy for operable breast cancer: A phase III randomized non-inferiority trial - The Lancet Regional Health – Western Pacific
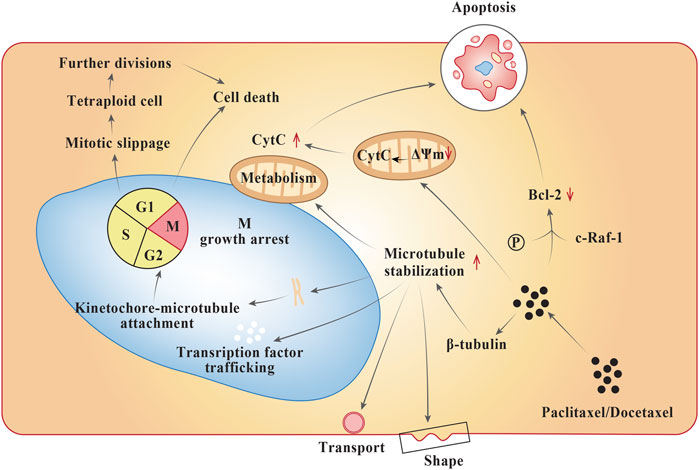
Frontiers | Platinum and Taxane Based Adjuvant and Neoadjuvant Chemotherapy in Early Triple-Negative Breast Cancer: A Narrative Review
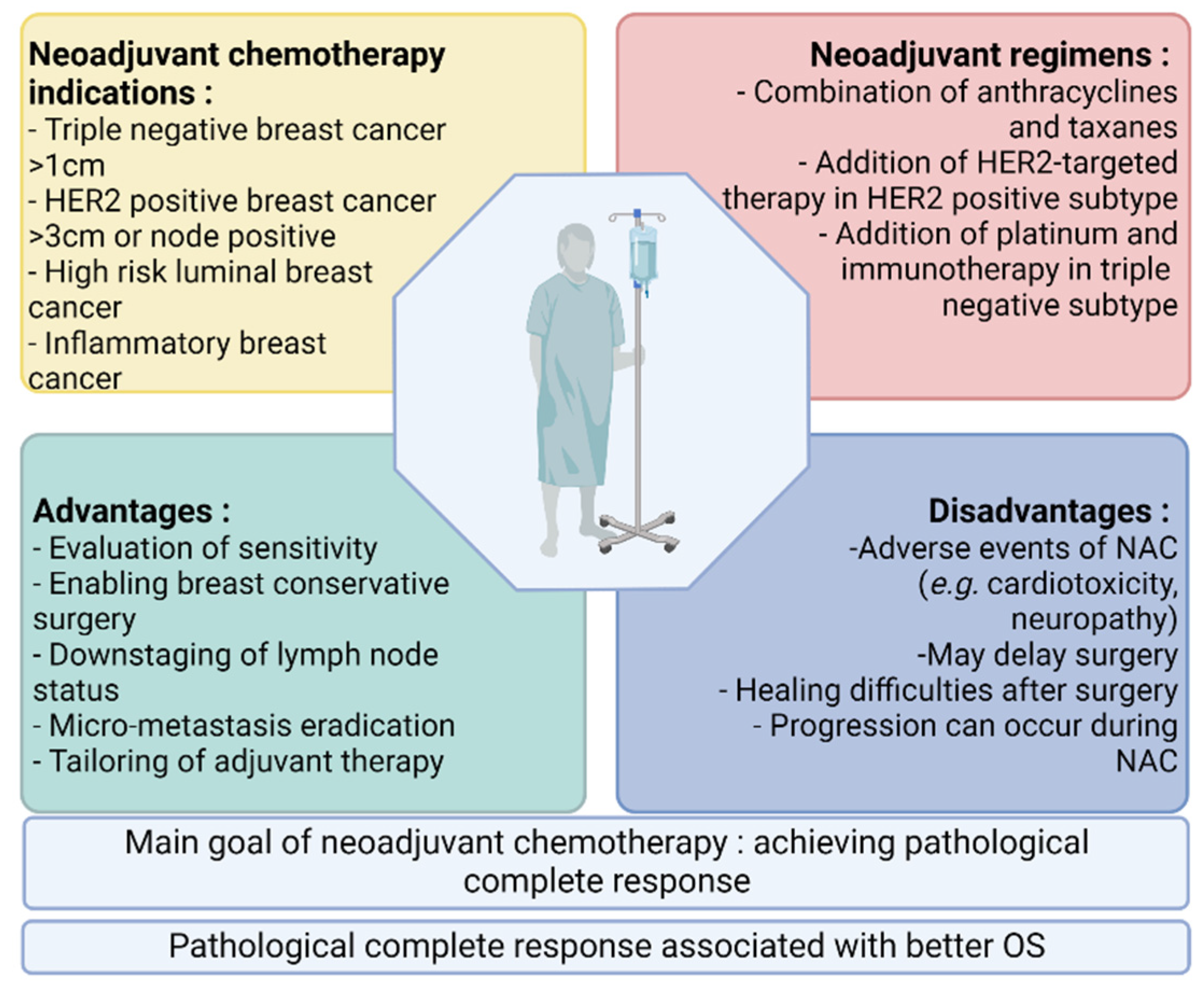
Cancers | Free Full-Text | Predictive Biomarkers of Response to Neoadjuvant Chemotherapy in Breast Cancer: Current and Future Perspectives for Precision Medicine

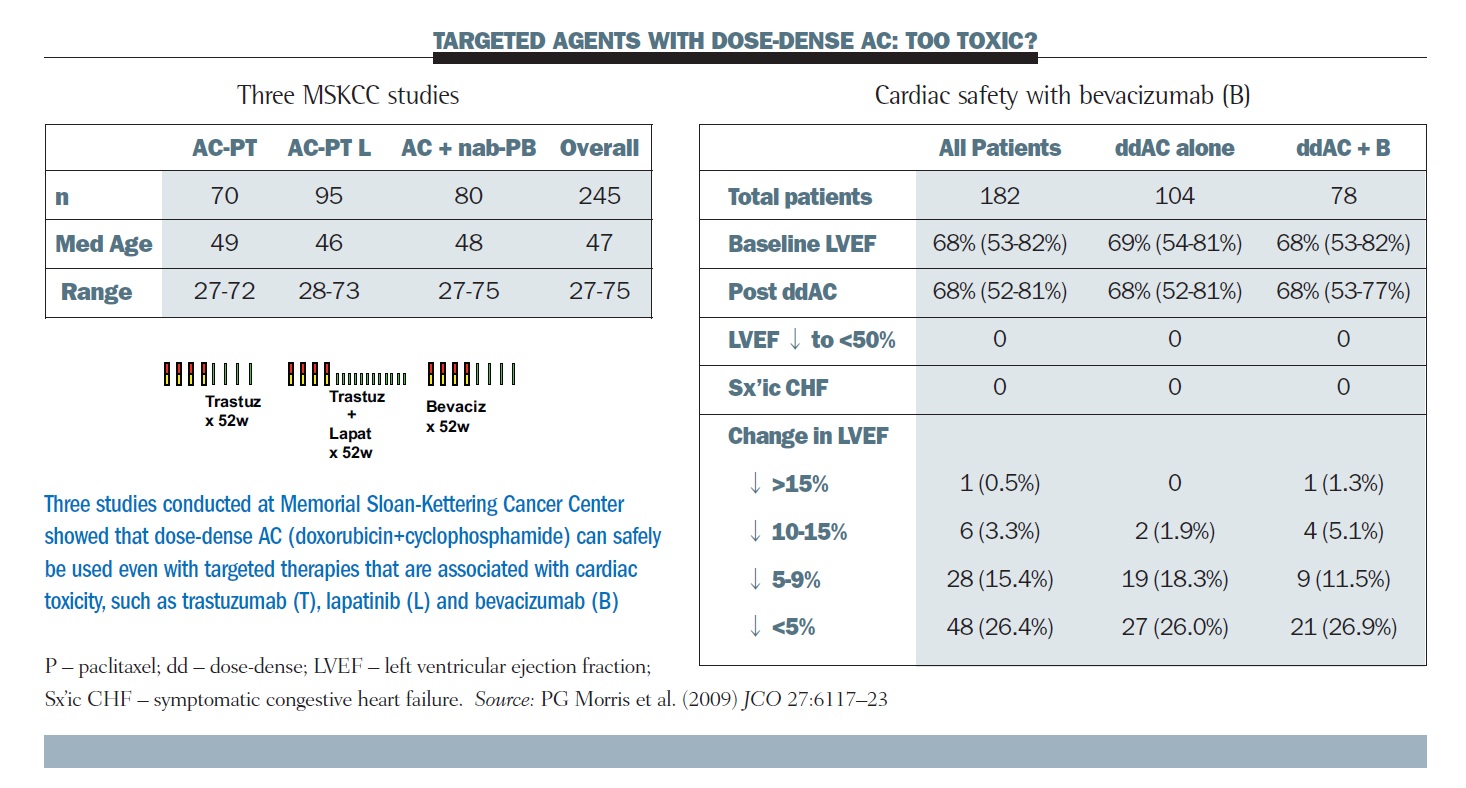
SWOG S 0800 ( NCI CDR 0000636131 ) : addition of bevacizumab to neoadjuvant nab-paclitaxel with dose-dense doxorubicin and cyclophosphamide improves pathologic complete response ( pCR ) rates in inflammatory or locally

Phase 2 Study of Dose-Dense Doxorubicin and Cyclophosphamide Followed by Eribulin Mesylate With or Without Prophylactic Growth Factor for Adjuvant Treatment of Early-Stage Human Epidermal Growth Factor Receptor 2–Negative Breast Cancer -

Standardizing Chemotherapy Regimen Nomenclature: A Proposal and Evaluation of the HemOnc and National Cancer Institute Thesaurus Regimen Content | JCO Clinical Cancer Informatics